Marine biofouling, the settlement of organisms on marine man-made structures, generates various and extensive challenges for industries, developing technologies, and working in the marine environment e.g., power plants, petro/chemical plants, refineries, LNG facilities, paper mills, steel mills and etc. Marine micro-organisms are diverse, molluscs, algae, or slime, and among them, those adhering to the seawater channels and equipment are mainly shellfish which cause fouling. In general, shellfish are said to start sticking when they are suspended in seawater in the age of larva when it is within several days after spawning. Pumps, filters, heat exchangers, channels, pipes, and vessels are highly prone to biofouling. This can adversely affect system hydrodynamics, restricting flow, increasing pumping pressures, and accelerating corrosion rate, which leads to higher operation and maintenance costs and shorter equipment lifetime.
It is widely known that continuous injection of a slight amount of chlorine is effective for preventing marine organisms’ build-up. Conventionally used, chlorine gas has several safety concerns, recognized as a potential terrorist target, and safety problem increases the likelihood of an accident/explosion.
The most common choice for switching from chlorine gas is commercial sodium hypochlorite, however, ordering a chemical in bulk necessitates the transfer and storage of large quantities of hazardous material. The decision process when evaluating alternatives to chlorine gas and commercial sodium hypochlorite focuses on safety, maintenance requirements, and costs.
A low-toxic and easy-to-use reagent, which has for sure been the leader among oxidants and disinfectants in the energy sector over the last decades is sodium hypochlorite generator. It is one of the safest, strongest, and most common forms of chlorine. Delivered hypochlorite (bulk bleach) is often perceived as safe, easy, and cheap. However, a proper evaluation shows that on-site generation of hypochlorite is not only safer than bulk bleach but also offers significant advantages and presents a return on investment within one to three years.
Electro-Chlorination system generates Sodium Hypochlorite, On-Site, and On-Demand, without the need for any additives or particular treatments. ULseawater™ and ULex™ systems have outstanding benefits in terms of safety, reliability, and stability of the system having a maintenance-free operation.
The electric current passes through the seawater or artificial brine solution by the UMMO™ Anodes. Now, the electrolysis generates the Sodium Hypochlorite that is the main product of the reaction. The hydrogen gas, as the by-product of the reaction, is going to be separated due to its lower density. The Electro-chlorination reaction is formulated as below:
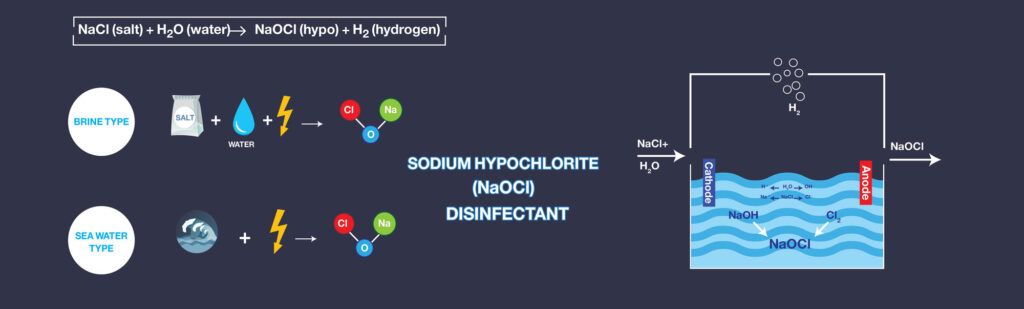
These reactions occur in Electrolyzers. Electrolyzer systems can be classified into two basic types, brine electrolysis, and seawater electrolysis. The basis for classification is the feedstock derived from either crystallized salt for brine systems or seawater feed for seawater electrolysis systems.
Although the product of each system is sodium hypochlorite disinfectant, differences in the electrolysis method exist as a result of the variations in the calcareous hardness and other properties of the feed material. Since crystallized salt is dissolved and used for electrolysis in brine systems, control of the calcareous components may be achieved using water softening or selecting the desired quality of the crystallized salt. Seawater does not allow for easy methods of calcareous component control. Thus, an entirely different approach to electrolysis is used in seawater systems.
To electrolyze a brine solution for sodium hypochlorite production, brine electrolysis cells are designed for very low brine feed flow rates with narrow electrode gaps that produce sodium hypochlorite concentrations approaching 8000 ppm (0.8%). The seawater system approach is to use very high seawater flow rates with wide electrode gaps that produce sodium hypochlorite concentrations up to 2000 ppm (0.2%) to reduce the rate of deposit formation on the cathodes. Hypochlorite is a powerful biocide and although it eventually decomposes back to harmless chloride ions and oxygen, it has the advantage of being relatively stable over other biocides.





